
29 3月 Flux Removes Lithium In Liquid Aluminium
Flux Removes Lithium In Liquid Aluminium is a solid substance (usually a mixture of chloride and fluoride) used in aluminum foundries. Its purpose is to reduce the oxidation of the melt, minimize the penetration of hydrogen, and absorb the suspended in the melt. Non-metallic inclusions, keep the furnace/ladle wall clear of accumulated oxides, reduce the aluminum content in the residue, remove the dissolved hydrogen in the melt, extract aluminum grains during the solidification process, and modify silicon inclusions in silicon-containing alloys , Oxidize excessive magnesium. The common practice of flux introduction is manual application.
Most of the flux is applied on the surface of the melt and stirred into the melt. Some flux (degassing, grain refinement) is cleaned into the bottom of the preheated perforated bell.
Flux can also be introduced into the melt by injecting it in powder form in a stream of inert gas (argon or nitrogen).
The simplest flux injection technique is to immerse the spray gun in the melt.
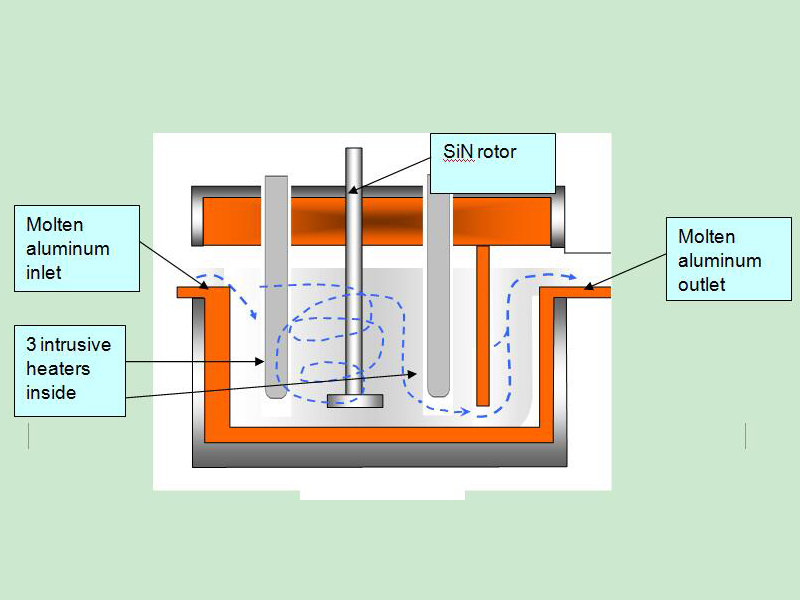
The most effective flux introduction method is to inject through a rotary degasser.
Classification of fluxes for melting aluminum
Tundish Covering Compound
Drossing fluxes
Cleaning fluxes
Wall cleaning fluxes
Degassing fluxes
Grain refiners
Silicon modifiers
Demagging fluxes

The problem of no flux in the furnace
When no flux is used in the furnace, the cleaning operation requires more force, which causes damage to the bricks.
If the furnace chamber is not well cleaned, oxides and spinel will accumulate on the furnace wall, especially at the metal wire.
The longer the oxide and spinel stick to the brick, the stronger it will stick until it is difficult to clean up.
At this time, the furnace must be thermally cleaned or cooled so that it can be mechanically cleaned with a jack hammer.
Both of these operations caused damage to the masonry and resulted in production losses.
According to reports, a foundry only uses flux-free mechanical cleaning, and the service life of the bricks is only 12 months.
The rebuilders of his furnace had to follow a regular smelting and cleaning schedule to ensure the lining.
Without flux, the metal is more contaminated by oxides and the product quality is worse.
There are several forms of fusion. Ideally, the flux is injected below the metal surface, where the flux melts and floats to the surface with oxides.
Then a semi-liquid is formed on the molten metal, which can effectively prevent further oxide contamination and reduce hydrogen absorption in the melt.
At the same time, the liquid salt softens the oxides on the furnace wall and reduces the bonding of oxides on the furnace wall.
Finally, the liquid in contact with the skimmed laver will wet the metal particles, bind them together, and then melt again.
In the holding furnace operation, applying flux in this way is the most effective.
In the smelting furnace, Flux Removes Lithium In Liquid Aluminium is often filled with solid metal to make it melt as the metal is smelted. During the smelting process, some salt will wet the surface and reduce oxidation.
Another application method is to spread flux on the surface of the skimming oil, where it melts and works downward through the skimming oil.
This is the most effective way to separate metals from oxidized laver.
The flux will cool the slag, which is a good insulator, so the flux will melt very slowly, unless the bricks have been added before the heating and burning cycle.
If the flux spreads in the combustion chamber, it will evaporate and produce dense smoke.
Since the flux is not left in the furnace, it is wasted.
Finally, the operator applies flux to the wall to soften the oxide and spinel so that they can be easily scraped off during the cleaning process.
Likewise, it is best to heat the refractory material before the flux operation, as the flux is generally more effective at higher temperatures. Cleaning should be done at the end of the combustion cycle.
The same principle applies to the crucible flux, but the flux can be added during the combustion cycle, as long as there is no flame directly hitting the flux.














No Comments