
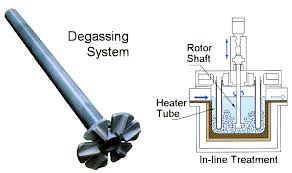
14 10月 Aluminium Degassing Unit
Aluminium Degassing Unit removes impurities from the melt to provide cleaner and better quality metals. It is one of the most common and effective cleaning methods used in foundries.
There are two main types of impurities found in molten aluminum: dissolved hydrogen and solids
Non-metallic inclusions. As the metal cools, the dissolved hydrogen escapes from the solution and forms harmful pores. This porosity and non-metallic solid inclusions reduce the strength and adversely affect the final performance of castings made of aluminum.

In aluminum, the reaction with water vapor is as follows:
·2 Al + 3 H2 O = Al2 O3 + 3 H2
Molecular hydrogen then dissociates in the molten metal:
·3 H2 = 6 hours
Molten aluminum also interacts with oxygen in the atmosphere, so in addition to the oxidation reaction shown in equation (a), the following reactions also occur:
·4 Al + 3 O2 = 2 Al2 O3
This reaction results in the formation of oxide scales on the surface of the molten metal during the melting process, and any subsequent transfer of the molten metal.
The oxides produced are trapped in most of the molten material and then transferred to the finished casting.
Other non-metallic inclusions, such as carbides, nitrides or borides, can come from sources such as crucible materials or other refractory materials.

Therefore, the Aluminium Degassing Unit must be used to remove dissolved hydrogen and non-metallic inclusions from the molten aluminum before casting to achieve the best quality.
The processes that have been developed for cleaning metals are physical processes that involve the use of inert gas fluxes.
The hydrogen dissolved in the molten material diffuses into the rising bubbles of the flux gas and is transported to the surface of the molten material. This process depends on two main steps:
The rate at which hydrogen diffuses through the diffusion boundary layer into inert bubbles
Concentration of hydrogen in inert bubbles
Diffusion is the degassing rate determination stage. Therefore, in order to achieve the best degassing efficiency, the following requirements must be met:
The bubbles of the inert gas are smaller and the residence time in the molten metal is longer. This ensures a large surface contact area between the inert bubbles and the molten metal, thus ensuring a higher mass transfer coefficient relative to the diffusion layer.
Contact sales@adtechamm.com to buy Degassing Unit.
The distribution range of inert bubbles on the entire cross section of the molten metal has been very wide
The full movement of the molten material accelerates the transmission of hydrogen to the inert bubbles
The static surface of the molten pool of molten material to avoid the absorption of fresh hydrogen from the reaction with the atmosphere.













Sorry, the comment form is closed at this time.